Abstract
Collective cell migration underlies morphogenesis, wound healing and cancer invasion1,2. Most directed migration in vivo has been attributed to chemotaxis, whereby cells follow a chemical gradient3,4,5. Cells can also follow a stiffness gradient in vitro, a process called durotaxis3,4,6,7,8, but evidence for durotaxis in vivo is lacking6. Here we show that in Xenopus laevis the neural crest—an embryonic cell population—self-generates a stiffness gradient in the adjacent placodal tissue, and follows this gradient by durotaxis. The gradient moves with the neural crest, which is continually pursuing a retreating region of high substrate stiffness. Mechanistically, the neural crest induces the gradient due to N-cadherin interactions with the placodes and senses the gradient through cell–matrix adhesions, resulting in polarized Rac activity and actomyosin contractility, which coordinates durotaxis. Durotaxis synergizes with chemotaxis, cooperatively polarizing actomyosin machinery of the cell group to prompt efficient directional collective cell migration in vivo. These results show that durotaxis and dynamic stiffness gradients exist in vivo, and gradients of chemical and mechanical signals cooperate to achieve efficient directional cell migration.
This is a preview of subscription content
Access options
Subscribe to Journal
Get full journal access for 1 year
199,00 €
only 3,90 € per issue
Tax calculation will be finalised during checkout.
Rent or Buy article
Get time limited or full article access on ReadCube.
from$8.99
All prices are NET prices.
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Source data are provided with the paper.
References
- 1.
Yamada, K. M. & Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019).
- 2.
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 (2009).
- 3.
Shellard, A. & Mayor, R. All roads lead to directional cell migration. Trends Cell Biol. 30, 852–868 (2020).
- 4.
SenGupta, S., Parent, C. A. & Bear, J. E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 22, 529–547 (2021).
- 5.
Insall, R. H. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat. Rev. Mol. Cell Biol. 11, 453–458 (2010).
- 6.
Shellard, A. & Mayor, R. Durotaxis: the hard path from in vitro to in vivo. Dev. Cell 56, 227–239 (2021).
- 7.
Lo, C. M., Wang, H. B., Dembo, M. & Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000).
- 8.
Sunyer, R. et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 353, 1157–1161 (2016).
- 9.
Charras, G. & Sahai, E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15, 813–824 (2014).
- 10.
Guimaraes, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
- 11.
Barriga, E. H., Franze, K., Charras, G. & Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527 (2018).
- 12.
Theveneau, E. et al. Chase-and-run between adjacent cell populations promotes directional collective migration. Nat. Cell Biol. 15, 763–772 (2013).
- 13.
Szabo, A. & Mayor, R. Mechanisms of neural crest migration. Ann. Rev. Genet. 52, 43–63 (2018).
- 14.
Shellard, A. & Mayor, R. Chemotaxis during neural crest migration. Semin. Cell Dev. Biol. 55, 111–118 (2016).
- 15.
Alfandari, D., Cousin, H., Gaultier, A., Hoffstrom, B. G. & DeSimone, D. W. Integrin α5β1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev. Biol. 260, 449–464 (2003).
- 16.
Wang, S. J. et al. Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J. Cell Biol. 199, 331–345 (2012).
- 17.
Schiller, H. B. et al. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625–636 (2013).
- 18.
Shellard, A., Szabo, A., Trepat, X. & Mayor, R. Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science 362, 339–343 (2018).
- 19.
Guilluy, C., Garcia-Mata, R. & Burridge, K. Rho protein crosstalk: another social network? Trends Cell Biol. 21, 718–726 (2011).
- 20.
Theveneau, E. et al. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell 19, 39–53 (2010).
- 21.
Koser, D. E. et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 19, 1592–1598 (2016).
- 22.
Zhu, M. et al. Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc. Natl Acad. Sci. USA 117, 4781–4791 (2020).
- 23.
Tse, J. R. & Engler, A. J. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. Plos ONE 6, e15978 (2011).
- 24.
Tweedy, L. et al. Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science 369, eaay9792 (2020).
- 25.
Dona, E. et al. Directional tissue migration through a self-generated chemokine gradient. Nature 503, 285–289 (2013).
- 26.
Thompson, A. J. et al. Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. eLife 8, e39356 (2019).
- 27.
Plotnikov, S. V., Pasapera, A. M., Sabass, B. & Waterman, C. M. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513–1527 (2012).
- 28.
Nieuwkoop, P. & Faber, J. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis 2nd edn (1967).
- 29.
Shi, J. L., Severson, C., Yang, J. X., Wedlich, D. & Klymkowsky, M. W. Snail2 controls mesodermal BMP/Wnt induction of neural crest. Development 138, 3135–3145 (2011).
- 30.
Tse, J. R. & Engler, A. J. in Current Protocols in Cell Biology Vol. 47 (Wiley Interscience, 2010).
- 31.
Yue, L., Wang, S., Wulf, V. & Willner, I. Stiffness-switchable DNA-based constitutional dynamic network hydrogels for self-healing and matrix-guided controlled chemical processes. Nat. Commun. 10, 4774 (2019).
- 32.
Giobbe, G. G. et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 10, 5658 (2019).
- 33.
Karoutas, A. et al. The NSL complex maintains nuclear architecture stability via lamin A/C acetylation. Nat. Cell Biol. 21, 1248–1260 (2019).
- 34.
Chen, C. Y. et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 24, 1225–1233 (2018).
- 35.
Ding, Y., Xu, G. K. & Wang, G. F. On the determination of elastic moduli of cells by AFM based indentation. Sci. Rep. 7, 45575 (2017).
- 36.
Gouignard, N., Rouvière, C. & Theveneau, E. in The Epithelial-to Mesenchymal Transition Vol. 2179 (eds Campbell, K. & Theveneau, E.) 257–274 (2021).
- 37.
Barriga, E. H., Shellard, A. & Mayor, R. in Neural Crest Cells Vol. 1976 (eds Schwarz, Q. & Wiszniak, S.) 135–152 (Humana, 2019).
- 38.
Dubaissi, E. et al. A secretory cell type develops alongside multiciliated cells, ionocytes and goblet cells, and provides a protective, anti-infective function in the frog embryonic mucociliary epidermis. Development 141, 1514–1525 (2014).
- 39.
Zhang, C., Basta, T., Jensen, E. D. & Klymkowsky, M. W. The beta-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130, 5609–5624 (2003).
- 40.
Schlosser, G. & Ahrens, K. Molecular anatomy of placode development in Xenopus laevis. Dev. Biol. 271, 439–466 (2004).
- 41.
Schlosser, G. et al. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev. Biol. 320, 199–214 (2008).
- 42.
Cao, X. et al. A phosphorylation switch controls the spatiotemporal activation of Rho GTPases in directional cell migration. Nat. Commun. 6, 7721 (2015).
- 43.
Kanoldt, V. et al. Metavinculin modulates force transduction in cell adhesion sites. Nat. Commun. 11, 6403 (2020).
- 44.
Karimi, K. et al. Xenbase: a genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Res. 46, D861–D868 (2018).
Acknowledgements
We thank G. Charras for the PDMS and discussions; J. Hartmann for assistance with calculating synergy statistics; E. Theveneau for gifting the Eya1 and Foxi1c probes; M. Klymkowsky for gifting the anti-Sox3 antibody; E. Barriga for preliminary experiments; and G. Charras, B. Stramer and J. Hartmann for comments on the manuscipt. Work in R.M.’s laboratory is supported by grants from the Medical Research Council (MR/S007792/1), Biotechnology and Biological Sciences Research Council (M008517, BB/T013044) and Wellcome Trust (102489/Z/13/Z).
Author information
Affiliations
Contributions
A.S. and R.M. conceived the project and designed the experiments. A.S. performed and analysed all of the experiments. R.M. contributed to experimental repeats and analysis of the data in Figs. 1c and 2b, e. A.S. and R.M. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
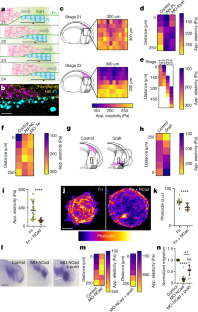
Extended Data Fig. 1 Neural crest use Placodes as substrate for migration.
a, Schematic of lateral view of an embryo showing the position of neural crest (pink) and placodes (blue) before neural crest migration. The black line represents the position of a cross section shown in c. b, Double in situ hybridization against the placodal marker Six1 and the neural crest marker Twist at pre-migratory stage23 Scale bar is 500 μm (b). c, e, Diagram illustrations of the neural crest environment in vivo, engaged in “chase and run”12 interaction with placodes. The diagram is illustrative of a cross-section through the embryo, has illustrated by the black line in a. Neural crest (pink) chemotax toward Sdf1-secreting placode (Sdf1, purple; placode, blue). The two cell types interact causing placode to run away. d, f, Cryosection images showing fluorescent in situ hybridization for Twist (neural crest, magenta), and immunostaining for Sox3 (placodes, cyan) and fibronectin (grey in single channel; yellow in merge). The fibronectin and merge panels are zooms of the white dashed boxes. Note that the neural crest migrates toward the placodes and that a fibronectin layer interfaces the two tissues. Scale bar is 50 μm (d, f). g, i, Fibronectin surround neural crest stream. g, Schematic of fibronectin (green) surrounding the neural crest at the interface with mesoderm and placodes. h, Double ISH (Twist) and immunostaining (fibronectin) at the interfaces shown in the squares in g. Scale bar is 20 μm (h) i, Quantification of fibronectin levels at the interface of neural crest with Placodes and Mesoderm. Note the higher levels of fibronectin at the interface of neural crest and placodes than with mesoderm. Thick bars (i) represent mean; error bars (i) represent s.d.; unpaired two-tailed t-test (i); ****P≤0.0001; n = 29 embryos each (i). Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 2 Dynamic stiffness gradient in placode cells.
a–b, Diagram illustrating removal of the epidermis to expose the placodes for atomic force microscopy (a). Cryosection images (b) showing that the placodes are the most superficial tissue after epidermis removal. The right merge panel is a zoom of the white dashed box. Scale bar is 50 μm (b). c, in situ hybridization against the placodal marker Eya1 at migratory stages on a representative embryo on the control side and side where the epidermis was dissected. Note that the placodal tissue is unaffected by epidermal dissection. Scale bar is 200 μm (c). d–g, Apparent elasticity measurements in vivo at the start of neural crest migration (c, d). Note that b and c represent the same data set. Stiffness measurements of the representative heat map in Fig. 1d, a stage 22 embryo (e) and where the epidermis was not removed (f). h–j, Placode deletion. h, Schematics of the different treatments. i, in situ hybridization against the placodal marker Eya1 after each treatment. Stiffness values after each treatment are shown in Fig 1d. Scale bar is 200 μm (i). j, in situ hybridization against the neural crest marker Twist in conditions stated. Note that neural crest migration depends on cranial placodes. Scale bar is 200 μm (j). k, Quantification of the distance of stiffness gradients measured by nanoindentation and cranial placode tissue in vivo. l–n, Diagram illustration of the neural crest and placodes in vivo (l). The area indicated represent the placode whose stiffness was measured after epidermis removal (not shown). Quantification of apparent elasticity measurements of the cranial placodes (m). Note that the gradient emerges at the onset of neural crest migration, and that the gradient persists as the neural crest migrate over time. A corresponding heat map representing averaged stiffness for embryos at different stages along the dorsoventral axis is sown in Fig 1e. Comparison of mesodermal and placodal stiffness over the dorsoventral axis (n). The dashed line indicates the rear of the placode. Thick bars (d, g, k, m, n) and circles (m) represent mean; error bars represent s.d. (d, g, k, m, n); Tukey’s test (d), Dunn’s test (g), two-tailed Mann-Whitney U test (k), Wilcoxon match-pairs signed rank test (m, st. 21), paired two-tailed t-test (m, st. 22, st. 23, st. 24); ns, P>0.05, ****P≤0.0001; n = 11 (d, e), 20 (g), 17 (k), 10 (m) embryos, 10 (f) linear lines. Diagrams in h are adapted from Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Copyright © 1994, Nieuwkoop and Faber. Reproduced by permission of Taylor and Francis books US. Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 3 The neural crest self-generates the stiffness gradient through N-Cadherin.
a, b, Schematics indicating the different treatments (a) and embryos stained by in situ hybridization for the neural crest marker, Slug (b). Heat maps of this experiments are shown in Fig. 1f. Scale bar is 200 μm (b). c, Embryos stained by in situ hybridization for the neural crest marker, Slug. Black boxes represent the region in which nanoindentation was performed; mean stiffness heat map of each condition is shown in Fig. 1h. Scale bar is 200 μm (c). d, Diagram illustrating “chase and run”12. Neural crest (pink) chemotax toward Sdf1-secreting placode (Sdf1, purple; placode, blue). The two cell types interact through N-Cadherin (green), causing placode to run away (migration indicated by black arrow). e, Diagram illustrating placode cultured on fibronectin (grey) or fibronectin with N-Cadherin. Placodal stiffness measured in these conditions is shown in Fig. 1i. f, Schematics indicating the different treatments. Results are shown in Fig. 1l–n. g, h, exogenous stiffness gradient formation. Stiffness heat map from a representative embryo in which an exogeneous local pressure was applied ventral to the neural crest as depicted in g (bottom of the heat map, g), and quantification along the axis (h). Thick lines (h) represent mean; error bars (h) represent s.d.; Dunn’s test (h); *P≤0.05, **P≤0.01; n = 4 (h) linear lines. Diagrams in a, f are adapted from Normal table of Xenopus laevis (Daudin). Copyright © 1994, Nieuwkoop and Faber. Reproduced by permission of Taylor and Francis books US. Statistics and reproducibility are in the source data and Methods
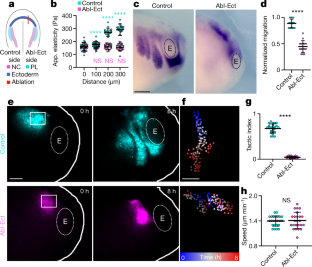
Extended Data Fig. 4 Neural crest durotaxis in vivo and on steep gradients ex vivo.
a, b, Graft of fluorescently labelled neural crest into control embryos (cyan) or ablated embryos (magenta; a, centred cell tracks; b, track angles). Grafted embryos are shown in Fig. 2d, e. c, Apparent elasticity measurements of steep stiffness (blue) and uniform (grey) gradient gels. d–g, Neural crest explants with labelled nuclei (magenta) and membrane (cyan) (d, f) and time-coded projected tracks (e, g) on gels of uniform stiffness (d, e) and graded stiffness (f, g). Scale bar is 50 μm (d-g). h–k, Cell tracks (h, j) and angles of movement (i, k) from clusters on gels of uniform (h, i) or graded (j, k) stiffness; l-n, Formula for tactic index (l), quantification of tactic index (m) and speed (n). Thick bars (c, m, n) represent mean; error bars (c, m, n) represent s.d.; two-tailed Mann-Whitney U test (m, n); ****P≤0.0001; n = 6 (c) gels, 360 (i, k) cells from 18 clusters, 17 (m, n) explants. Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 5 Rear actomyosin contraction is essential for collective durotaxis.
a, Neural crest in vivo expressing fluorescently tagged myosin light chain II (MLC) and LifeAct. Note that a supracellular actomyosin cable exists at the edge of the neural crest cell group in vivo. Arrowheads indicate the actomyosin cable. Scale bar is 25 μm (a). b, Kymograph of the edge of the neural crest cluster in vivo. Green represents MLC, which is absent from cell-cell contacts, and red represents LifeAct. Note the in vivo contraction of the actomyosin cable. Cell-cell contact contraction is indicated with the black arrowheads. c, Neural crest ex vivo expressing fluorescently tagged myosin (MLC, myosin light chain), LifeAct and membrane marker. Corresponding low magnifications are shown in Fig.4a. Scale bar is 25 μm (c). d, Two time points from the edge of a neural crest cluster on a stiffness gradient. Yellow arrowheads mark cell-cell contacts. Note the contraction of the actomyosin cable and reduction in length between cell contacts. Scale bar is 10 μm (d). e, Heat map of an example actomyosin contraction at the edge of clusters in control, durotaxis and chemotaxis. Time point zero represents the start of actomyosin contraction. f, Quantification of actomyosin contraction by cable length. Note that the amplitude of contraction is the same in all conditions. g, h, An illustrative diagram showing rear contraction and front movement of a cluster (circle outline) at three time points (g), and a histogram representing the time at which front movement occurs relative to rear contraction (h). The dashed line indicates the rear contraction time point reference, t = 0. i, j, Tactic index (i) and speed (j) of clusters on shallow stiffness gradient gels exposed to the myosin II inhibitor, blebbistatin. k-m, Pictures of membrane and merge of LifeAct and membrane for the example ablation shown in Fig. 3c–g (k, top, middle) or for cytoplasmic ablation (k, bottom). Yellow arrowheads indicate location of ablation. Scale bar is 10 μm (k). l, Migration of neural crest clusters on physiological stiffness gradients. The dashed line (start of ablations) separates before and during laser ablation of the cytoplasm. m, Tactic index of clusters before and during actomyosin cable ablation in the front or rear portion of migrating cell groups, or in the cytoplasm. Thick bars (f, i, j, l, m) represent mean; error bars (f, i, j, l, m) represent s.d.; unpaired two-tailed t-test (i), two-tailed Mann-Whitney U test (j), Dunn’s test (m); ns, P>0.05, *P≤0.05, ****P≤0.0001; n = 20 (f), 15 (h), 30 (i, j), 6 (l, m) clusters. Statistics and reproducibility are in the source data and Methods
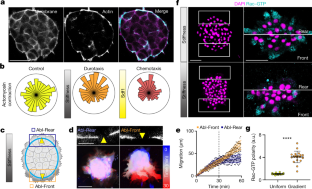
Extended Data Fig. 6 Rac during durotaxis.
a–b, Cells at the front and rear of neural crest explants on a stiffness gradient, with Rac-GTP, Phalloidin and DAPI labelling (a), and quantification of Rac-GTP along the axis from the edge of the cluster inwards (b). Note that Rac-GTP preferentially accumulates at the cell edge irrespective of its position within the cluster, consistent with previous observations of Rac polarity. Scale bar is 10 μm (a). c, d, Immunostaining of vinculin with Phalloidin in explants on uniform stiffness (c, top; m) or a physiological stiffness gradient (c, bottom) and polarity of the number of vinculin spots quantified (d). Scale bar is 5 μm (c). e, f, Immunostaining of Rac-GTP in control or Integrin-β1 knockdown (e) and quantification of its polarity (f). Scale bar is 50 μm (e). Thick bars (b, d, f) represent mean; error bars (b, d, f) represent s.d.; two-tailed Mann-Whitney U test (d, f), ****P≤0.0001; n = 20 (b, d, f) clusters. Statistics and reproducibility are in the source data and Methods
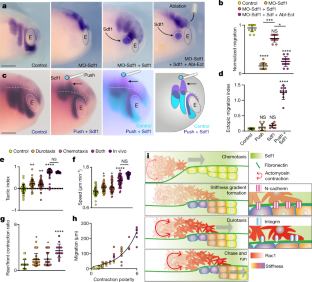
Extended Data Fig. 7 Durotaxis and chemotaxis cooperatively coordinate neural crest migration in vivo.
a, Schematics indicating the different treatments. Results are shown in Fig. 4a, b. b, Stiffness measurements in control and ablated embryos. Quantification of in vivo migration in each condition is shown in Fig 5b. c, d, An example heat map of stiffness from local pressure treatment (c) as depicted in Fig. 4c and quantification of the exogeneous stiffness gradient (d). Results are shown in Fig. 4c. e–l, Ectopic migration analysis. e, Schematic illustrating how the ectopic migration index (emi) was calculated. For each neural crest stream a vector was drawn from their origin to the final position of migration. The control and experimental side of the same embryos were analysed. For the control side the vector always lays in the migratory pathway, while for the experimental side some vectors point to ectopic locations. f, The difference between these two vectors \(\mathop}\limits^{\rightharpoonup },\mathop}\limits^{\rightharpoonup }\) generates the ectopic migration, which normalized by the control vector corresponds to the emi, which is shown as an scalar value in Fig. 4d. g, h, emi vectors for the experiment described in Fig. 4c. i–l, In situ hybridization for Twist of control and experimental side of embryos treated with exogenous local pressure (i, j) or an SDF1 bead (k, l) and the associated vectors. Scale bar is 250 μm (i, k). Thick bars (b, d) represent mean; error bars (b, d) represent s.d.; Tukey’s test (b, control; d), Dunn’s test (b, ablation); ns, P>0.05, *P≤0.05, ***P≤0.001, ****P≤0.0001; n = 11 (b, control), 10 (b, ablation), 12 (d), 9 (g, h, j, l) embryos. Diagrams in a are adapted from Normal table of Xenopus laevis (Daudin). Copyright © 1994, Nieuwkoop and Faber. Reproduced by permission of Taylor and Francis books US. Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 8 Fabrication of physiological stiffness gradient gels.
a, Apparent elasticity measurements of shallow (physiological) stiffness gradient gels and uniform stiffness gels. b, Gradient of stiffness from in vivo embryo measurements and ex vivo polyacrylamide gels. c–d, Immunostaining of fibronectin and fluorescent microspheres in gel. Images represent soft and stiff sides of the same gel exhibiting a stiffness gradient, in either top view (c) or side view (d). Scale bar is 100 μm (c, d). e–f, Quantification of fibronectin thickness (e) and mean fluorescence (f). g, Tactic index of clusters seeded on different regions of physiological gradient gels. h–j, Immunostaining of Rac-GTP of clusters on a stiffness gradient or uniformly high stiffness (h), quantification of total Rac-GTP (i) and Rac-GTP polarity (j) on different portions of the gradient gel. Scale bar is 20 μm (h). Thick bars (a, b, e-g, i, j) represent mean; error bars (a, b, e-g, i, j) represent s.d.; unpaired two-tailed t-test (b, e), two-tailed Mann-Whitney U test (f), Dunn’s test (g, i, j); n = 8 (a), 30 (e), 20 (f), 28 (g, 0.6, 1.4), 25 (g, 1), 33 (g, 1.8) gels, 15 (b) clusters and embryos, 29 (i, j) clusters. Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 9 Synergistic effects of chemotactic and durotactic gradients and comparison of collective versus single cell durotaxis.
a–i, Time-coded projected tracks of example clusters (a, c, e, g), angles of the tracks (b, d, f, h), quantification of directional migration (i). Scale bar is 100 μm (a, c, e, g). j–m, Synergy analysis, real combination of chemotaxis and durotaxis is compared with the inferred combination. Clusters exposed to durotactic and chemotactic gradients simultaneously and the inferred addition of track angles (j), speed (k), tactic index (l), and migration distance (m) of clusters exposed to durotactic and chemotactic gradients simultaneously based on the data with either gradient alone. n, A time-coded projected track of an individually migrating neural crest cell on a physiological stiffness gradient. Scale bar is 40 μm (n). o, A circular histogram showing the angles of the tracks by single cells plated on physiological (shallow) shallow stiffness gradients. p, Quantification of tactic index by clusters and single cells on physiological (shallow) stiffness gradients. Box plots (k-m) show the median, box edges represent the 25th and 75th percentiles, and whiskers show spread of data; thick bars (p) represent mean; error bars (p) represent s.d.; resampling test (j-m), two-tailed Mann-Whitney U test (p); *P≤0.05, ***P≤0.001, ****P≤0.0001; n = 42 (a, real combination), 600 (a, inferred combination; b, inferred combination), 94 (b, real combination), 16 (c, real combination), 570 (c, inferred combination), 43 (p, cell cluster) clusters, 600 (o) angles, 44 (p, single cell) cells. Statistics and reproducibility are in the source data and Methods
Extended Data Fig. 10 Chemotaxis and Durotaxis synergically control actomyosin contraction polarity.
a, Heat maps derived from the change in actomyosin cable length. Actomyosin contraction pulses are cyan/purple rectangles. Note that front contractions are inhibited when clusters are exposed to chemical and mechanical gradients. b, Quantification of the frequency of actomyosin contractions at the rear and front of cell clusters in control (purple), durotaxis (lilac), chemotaxis (green) and both (blue). Thick bars represent mean (b); error bars represent s.d. (b); Dunn’s test (b); ns, P>0.05, *P≤0.05, ***P≤0.001, ****P≤0.0001; n = 32 (b, control rear), 35 (b, control front), 41 (b, durotaxis rear), 26 (b, durotaxis front), 29 (b, chemotaxis rear), 28 (b, chemotaxis front), 25 (b, both rear), 26 (b, both front) clusters. Statistics and reproducibility are in the source data and Methods
Supplementary information
Supplementary Video 1
Stiffness gradient ablation inhibits neural crest migration in vivo. Fluorescently labelled neural crest grafts in a control host embryo (left) and host embryo that is mechanically ablated to abrogate the stiffness gradient (right).
Supplementary Video 2
Stiffness gradient ablation inhibits directional neural crest migration in vivo. Fluorescently labelled neural crest grafts in a control host embryo (left) and host embryo that is mechanically ablated to abrogate the stiffness gradient (right).
Supplementary Video 3
Neural crest durotaxis ex vivo. Neural crest clusters expressing fluorescent nuclei (magenta) and membrane (cyan) markers explanted on a gel of uniform (left) and steep gradient (right) stiffness.
Source data
Rights and permissions
About this article
Cite this article
Shellard, A., Mayor, R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature (2021). https://ift.tt/31FIwCY
-
Received:
-
Accepted:
-
Published:
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"along" - Google News
December 08, 2021 at 11:07PM
https://ift.tt/31FhxHh
Collective durotaxis along a self-generated stiffness gradient in vivo - Nature.com
"along" - Google News
https://ift.tt/2z4LAdj
https://ift.tt/35rGyU8
Bagikan Berita Ini















0 Response to "Collective durotaxis along a self-generated stiffness gradient in vivo - Nature.com"
Post a Comment